NICE backs Novartis' Adakveo via special channel despite 'high uncertainty' about cost, long-term efficacy | Fierce Pharma
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy - Frazer A. Tessema, Jonathan J. Darrow, 2017
A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy | Journal of Law, Medicine & Ethics | Cambridge Core
A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy - Frazer A. Tessema, Jonathan J. Darrow, 2017
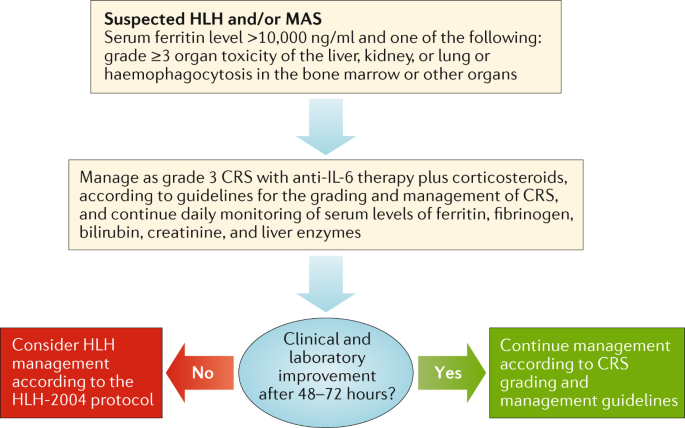
Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy | Nature Reviews Clinical Oncology

ASCO: Gilead's Kite soars over Novartis' CAR-T turf with Tecartus win in type of leukemia | Fierce Pharma
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
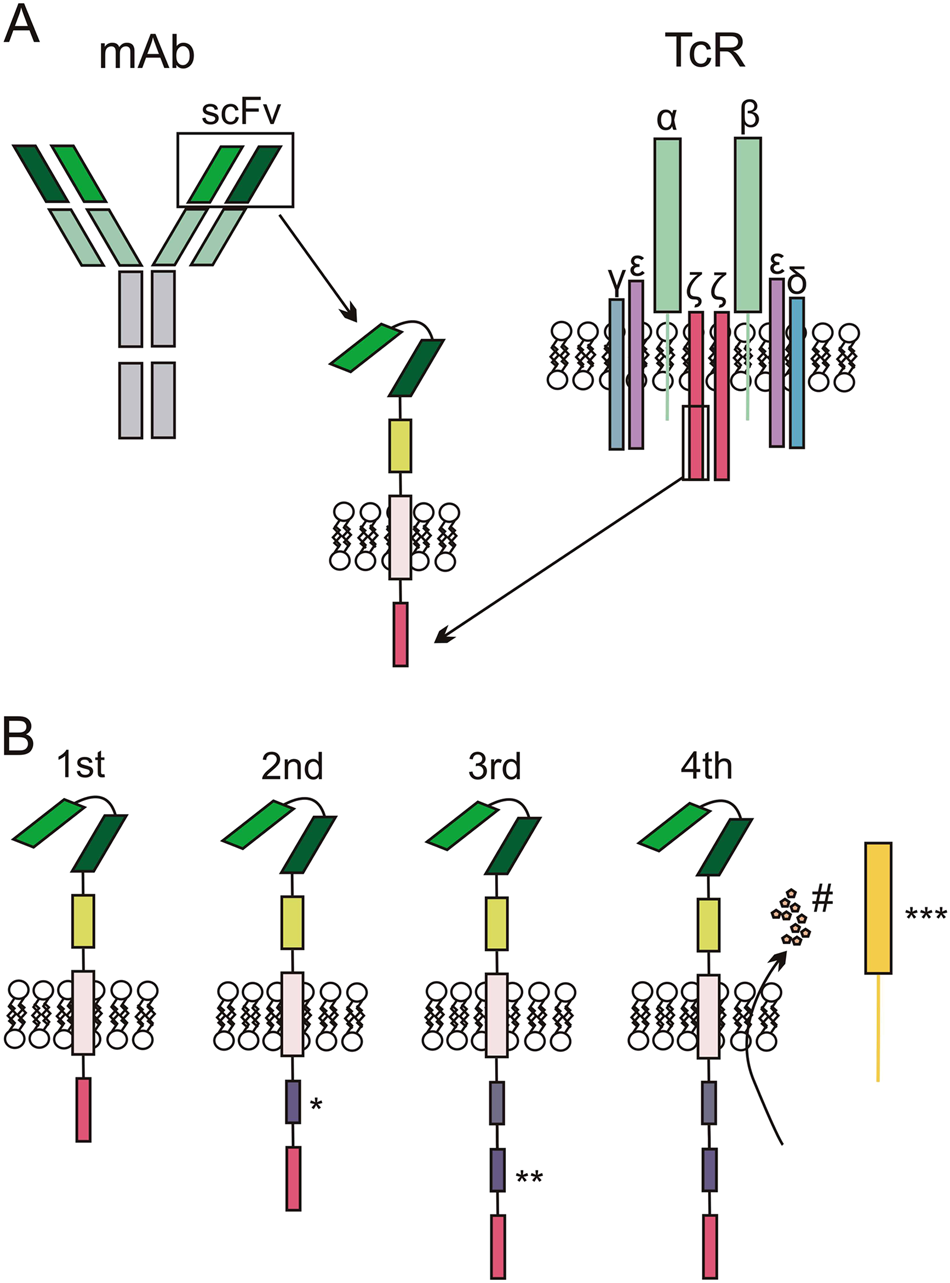
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease
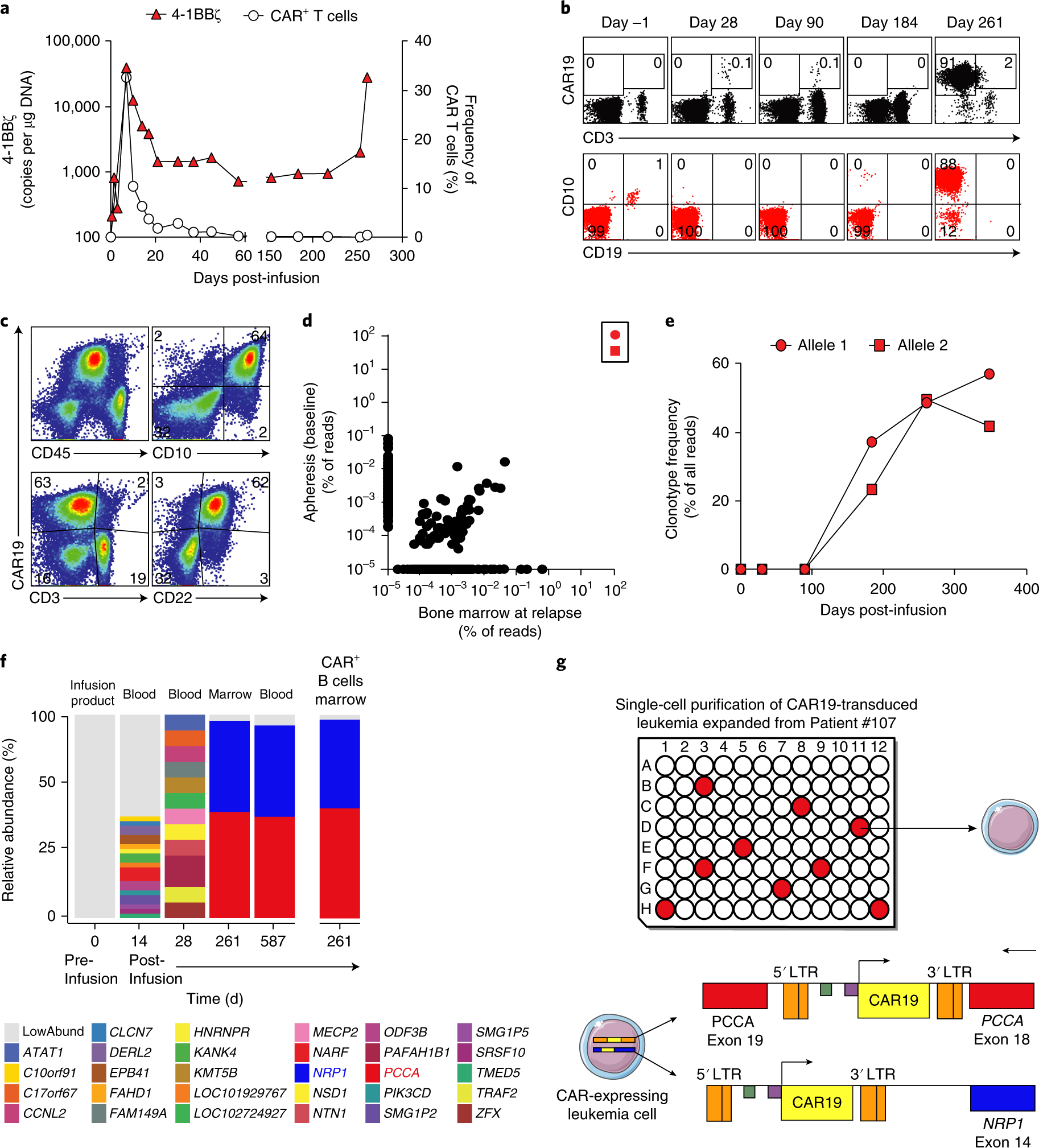
Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell | Nature Medicine

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
ONCOLOGIC DRUGS ADVISORY COMMITTEE BRIEFING DOCUMENT Tisagenlecleucel (CTL019) for the TREATMENT OF PEDIATRIC AND YOUNG ADULT PA

Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas | Nature Medicine
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
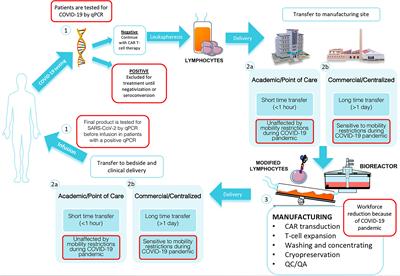
Frontiers | Manufacturing and Management of CAR T-Cell Therapy in “COVID-19's Time”: Central Versus Point of Care Proposals

Can CAR-T and gene therapy cures really sustain biopharma? Not for all, analyst says | Fierce Pharma

Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma | Nature Communications
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19 - Cytotherapy